背景及概述[1]
对硝基基-β-D-吡喃葡糖醛酸苷为糖类衍生物,可用作医药中间体。
制备[1]
对硝基基-β-D-吡喃葡糖醛酸苷制备具体步骤如下:
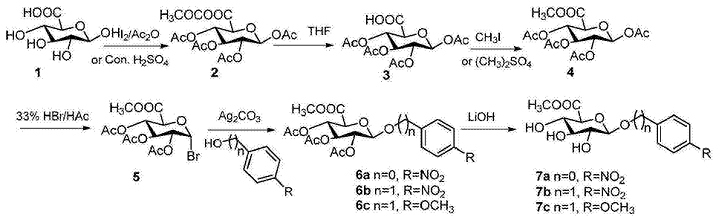
1)1,2,3,4-四-O-乙酰基葡萄糖醛酸酐(2)的制备
在氮气保护下,20克β-D-葡萄糖醛酸(1)(103.0mmol)溶于100mL乙酰酐中,8滴浓滴入,反应升温至60℃,继续反应1小时,降至室温,减压浓缩,蒸出酐至1/4,加人,白色固体析出,真空干燥得39.3g五乙酰葡萄糖醛酸酐,产率为94.3%。或者,加入催化量的碘替代浓,在40℃下搅拌反应2小时,采用同样的后处理,得38.4g五乙酰葡萄糖醛酸酯,产率92.1%。1HNMR(400MHz,DMSO-d6)δ:5.99(d,J=8Hz,1H),5.46(t,J=12,8Hz,1H),5.04(t,J=12,8Hz,1H),4.94(t,J=12,8Hz,1H),4.51(d,J=12Hz,1H),2.07(s,3H),2.0(s,3H),1.97(s,3H),1.96(s,3H),1.90(s,3H).13CNMR(100MHz,DMSO-d6)δ:172.46,169.88,169.50,169.10,168.36,91.01,72.02,71.58,70.26,69.13,21.48,20.88,20.75,20.72,20.70.
2)1,2,3,4-四-O-乙酰基-D-葡萄糖醛酸(3)的制备
在氮气保护下,21g1,2,3,4-四-O-乙酰基-葡萄糖醛酸酐(2)(51.94mmoL)溶于150mL四氢呋喃与水的混合溶液(1:1=V/V),搅拌过夜,减压蒸除四氢呋喃,过滤,真空干燥得18.0g白色固体,产率95.7%.1HNMR(400MHz,DMSO-d6)δ:13.47(brs,1H),5.99(d,J=8Hz,1H),5.46(t,J=12,8Hz,1H),5.05(t,J=12,8Hz,1H),4.95(t,J=12,8Hz,1H),4.51(d,J=8Hz,1H),2.07(s,3H),2.00(s,3H),1.97(s,3H),1.96(s,3H).13CNMR(100MHz,DMSO-d6)δ:169.88,169.50,169.17,168.35,91.01,72.01,71.58,70.26,69.13,20.88,20.75,20.71,20.70.
3)1,2,3,4-四-O-乙酰基-D-葡萄糖醛酸甲酯(4)的制备
在氮气保护下,19.2g四乙酰-O-葡萄糖醛酸(3)(52.99mmol)溶于200毫升干燥的N,N-二甲基甲酰胺,29.0g碳酸钾(204.32mmol)和12.0g碘甲烷(84.48mmoL)慢慢加入,继续搅拌2小时,倾倒入800g冰水中,剧烈搅拌,过滤,蒸馏水洗涤,滤饼真空干燥得白色固体四乙酰-O-葡萄糖醛酸甲酯19.2g,产率96.4%。1HNMR(400MHz,CDCl3)δ:5.77(d,J=8Hz,1H),5.30(t,J=12,8Hz,1H),5.23(t,J=12,8Hz,1H),5.13(t,J=12,8Hz,1H),4.18(d,J=8Hz,1H),3,74(s,3H),2.11(s,3H),2.03(s,3H),2.02(s,3H).13CNMR(100MHz,CDCl3)δ:169.88,169.38,169.15,168.80,166.76,91.31,72.94,71.77,70.09,68.87,53.00,20.75,20.54,20.51,20.44.
4)2,3,4-三-O-乙酰基-α-D-溴代葡萄糖醛酸甲酯(5)的制备
在氮气保护下,将25.6克1,2,3,4-四-O-乙酰基-β-D-葡萄糖醛酸甲酯(4)(68.0mmol)溶入120毫升二氯甲烷中,冷却至0℃,滴加150mL33%HBr的醋酸溶液,此温度下继续搅拌2小时,TLC监测,完毕,加入水稀释,用二氯甲烷萃取,有机层用饱和碳酸氢钠、饱和食盐水洗涤,无水钠干燥,过滤,减压浓缩,硅胶柱层析纯化粗品,流动相为石油醚:(4::1,V/V),得白色固体22.7克,产率为83.9%。1HNMR(400MHz,CDCl3)δ:6.51(d,J=4Hz,1H),5.44(t,J=12,8Hz,1H),5.09(t,J=12,8Hz,1H),4.73(t,J=12,8Hz,1H),4.42(d,J=12Hz,1H),3,61(s,3H),1.95(s,3H),1.91(s,3H),1.90(s,3H).13CNMR(100MHz,CDCl3)δ:169.40,169.38,169.20,166.44,85.54,71.89,70.06,69.09,68.24,52.90,20.37,20.22.
5)对硝基基-2,3,4-三-O-乙酰基-β-D-葡萄糖醛酸甲酯糖苷(6a)的制备
在氮气保护下,20.8克碳酸银(75.6mmoL)、5.3克(37.8mmoL)对硝基酚、催化量的碘(0.3g)分别溶入40mL二氯甲烷,加入分子筛,搅拌10mins,溶有10克2,3,4-三-O-乙酰基α-D溴代葡萄糖醛酸酯(5)(25.2mmoL)10毫升二氯甲烷缓缓加入,滴毕,用锡箔纸包裹反应24小时,加入稀释,硅藻土过滤,滤液减压浓缩,粗品硅胶柱纯化,用石油醚与(5:1,V/V)制备10.0克白色固体,产率88.1%。1HNMR(400MHz,CDCl3)δ:8.18(m,2H),7.07(m,2H),5.38-5.28(m,4H),4.26(m,1H),3.71(s,3H),2.06(s,3H),2.05(s,3H),2.04(s,3H).13CNMR(100MHz,CDCl3)δ:169.99,169.34,169.13,166.58,161.02,143.29,125.81,116.58,98.05,72.57,70.56,68.69,20.58,20,59,20.47.
6)对硝基苄基-β-D-葡萄糖醛酸糖苷(7a)的制备
在氮气环境中,将17.8克对硝基基2,3,4-三-O-乙酰基-β-D-葡萄糖醛酸甲酯糖苷(6a)(40mmol)溶液无水甲醇溶液中,加入4.8克氢氧化锂(200mmoL),室温搅拌4小时,TLC监控进程,Dowex50WX树脂加入中和过量碱,过滤,浓缩得对硝基基-β-D-葡萄糖醛酸糖苷,用乙醇重结晶得10.7克白色片状的固体,产率85%。
1HNMR(400MHz,D2O)δ:8.15(m,2H),7.14(m,2H),5.20(d,J=8Hz,1H),4.06(t,J=12Hz,1H),3.59(dd,J=8,6.0Hz,1H),3.56(d,J=12.0Hz,6.0Hz,1H),3.55(dd,J=8.0,6.0Hz,1H).
主要参考资料
[1] CN201810503213.4一种取代苄基或取代基β-D-己糖醛酸糖苷的制备方法




